
= Valence electrons of Sulfur + Valence electrons of Fluorine Therefore The total number of valence electrons in this compound comes to be, Apart from this, there are 4 other Fluorine atoms.Īs there are four atoms of Fluorine, the total number of valence electrons of Fluorine are = 4*7 = 28 The central atom in this compound is Sulfur.
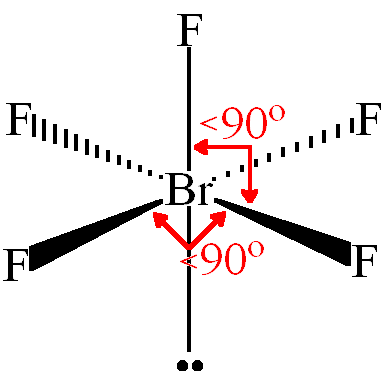
To draw the structure of SF4 we will need to know the total number of valence electrons that exist in SF4. While the valence electrons which do not participate in any bonding are represented using dots. The bonds that are formed between two atoms are represented using lines. Let us look at how SF4’s Lewis structure can be formed. This is the general idea of how and why Lewis structures are made.
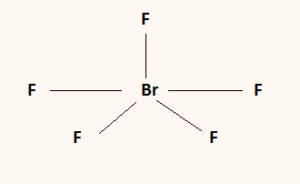
Here the central atom is Sulfur and the neighboring atoms are Fluorine. The Lewis structure helps one to understand the sharing of electrons between the central and neighboring atoms within a compound. And the valence electrons that do form bonds are known as bonding pairs of electrons. The valence electrons that do not form bonds are known as non-bonding pairs of electrons. Within a Lewis Structure, atoms come together as either lone electrons or by forming a bond. If you want to understand the properties of any compound better then you have to study its Lewis structure.
#Brf5 molecular geometry how to#
In a Nutshell How to Draw Lewis Structure Now when we know the basic things about the compound, we should move to see other properties of SF4 like polarity, geometry, and more. SF4 has the potential to cause serious damages as well as problems like cancer but the data has not been accessed fully to be termed as true. Workers who get exposed to vapors of this compound may experience shortness of breath, headache, eye and nose irritation, vomiting, and more. Otherwise, it is a hazardous compound, capable of strong reactions. The compound is assumed to be stable only when the right storage conditions are met.
When SF4 gets exposed to extreme heat, there are chances that the container with this compound may burst. The same happens when it reacts with some acid. If it gets reacted with water then toxic fluoride and sulfur oxide fumes are formed along with an acidic solution. SF4 is a toxic gas if inhaled and can cause serious irritation in the skin, eyes, or mucous membrane. SF4’s boiling and melting points are -38 degrees Celcius and -121 degrees Celcius respectively. The molecular weight of this compound is calculated to be 108.6 g/mol. This compound is generally identified as being a colorless gas. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs.


 0 kommentar(er)
0 kommentar(er)
